This page was created as an assignment for Genetics 677, an undergraduate course at UW-Madison.
Nuclear comparison of HGPS child and healthy mother
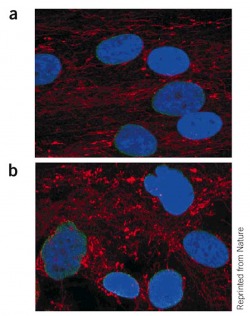
Figure 1. (a) Skin fibroblasts from a mother with a normal LMNA sequence and (b) her affected child, a heterozygote for the most common LMNA mutation in HGPS (G608G).
Lamins A and C in green, nuclei in blue, and mitochondria, to show the distribution of cytoplasm, in red. About half of the nuclei from the HGPS subject had an irregular shape.
Gene found for rapid aging disease in children
One week before the discovery of the gene that causes Hutchinson-Gilford Progeria Syndrome was published in Nature , Steve Sternburg reported the findings in USA Today. Sternburg announced the news that French and American research groups identified the genetic link causing HGPS. The discovery came just a year after the American team began the search for the cause of HGPS. The quickness of these findings is surprising as the disease is so difficult to study as only a dozen children afflicted with this disease are alive at any given time in the US. The US study was led by Francis Collins of the National Human Genome Research Institute, who used "an astonishing array of genetic research tools" to discover the gene linked to HGPS. Researchers found that in 18 of the 20 patients studied there was a common base substitution in one gene that is thought to cause HGPS. The mutated gene codes for lamin A, an integral protein for the nuclear membrane. Also included in the press release was a statement from John Tacket, the oldest living person at age 15, with HGPS in 2003.
The article by Sternburg is a fairly strong summary of the discovery and makes it comprehensible to the general public. The mutation leading to HGPS is not very complex, as it is a very short network which leads to the disease. He discusses the the single base "misspelling" in a chromosome that is the basis for a mutation in the gene that produces lamin A. He also goes on to describe the role of lamin A in cells, and that 50% of those with HGPS show abnormalities in cell nuclear shape. Overall he gives an understandable summary of the research group's discovery. However, he lacks in showing the number of different experiments used in identifying this genetic link by using a blanket statement saying that an "astonishing" number of genetic tools were used. Also from the article it appears the gene causing HGPS was found simply by looking at genome data. Both of these are over-simplified and lose the context of this discovery. There was as many, if not more, conventional molecular experimentation and research applied to this problem than genomic sequencing. Also, the scientists pinpointed the exact cause, a 50 amino acid deletion on exon 11 of the LMNA gene. Though this is too technical for popular press Sternburg could have done a better job detailing the findings and the root cause of HGPS. Also he describes the point mutation as being in the genetic code of a chromosome, and he then quickly jumps to talking about genes. It would be better to detail that genes are located on chromosomes and give some background about this. Doing so may give the reader a better background on the molecular basis behind the disease. When dealing with such technical science discoveries it can be difficult to convey the findings in a complete manner while also catering to a popular audience.
Article reviewed was from USA Today by Steve Sternberg.
Journal article describing actual discovery can be found here: doi: 10.1038/nature01629
Figure 1. Hegele, R A. (2003). Lamin mutations come of age. Nature Medicine, 9, 644-645. doi: 10.1038nm-0603-644
Peter St. Andre [email protected] Last updated:2/12/09
gen677.weebly.com
